Stability Study Protocol Template. Stability study protocol template pharmaceutical objective of study this protocol is prepared to carry out stability study of process validation and ongoing batches of aspirin 75 mg tablets batch size 100000 tablets as per ich guideline 6 3 stability protocol template table labware 6 3 stability protocol template table 6 3 1 stability protocol template concepts a stability protocol template is the generic definition of a stability protocol and is used as the basis for creating stability. Sorry if I come off rude but since this is your.
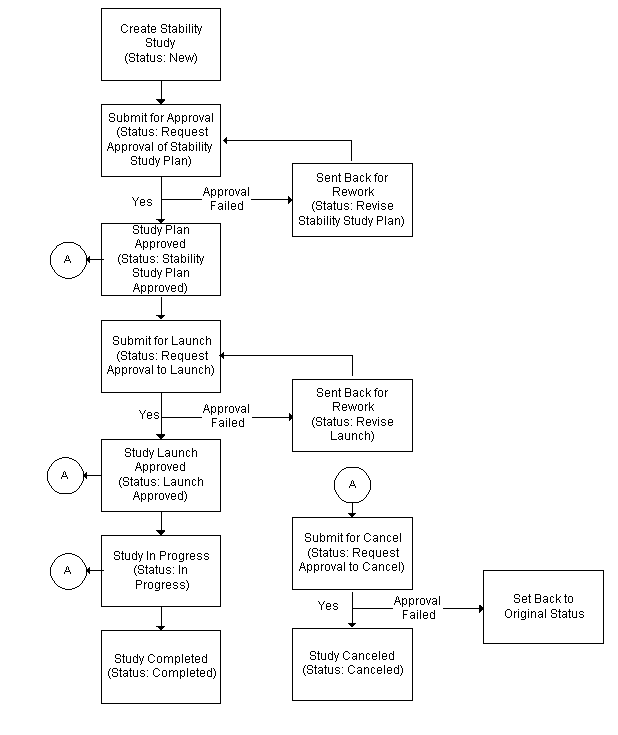
Can anyone provide me a detail stability study protocol templet for new product please. Lisinopril and Hydrochlorothiazide tablets 10125 mg CONTENTS. After implementing the improvements suggested by this project the new process cut the median stability protocol planning cycle time in half and reduced losses by 130000.
After implementing the improvements suggested by this project the new process cut the median stability protocol planning cycle time in half and reduced losses by 130000.
Can anyone provide me a detail stability study protocol templet for new product please. The purpose of this Standard Operating Procedure SOP is to describe the procedure for the preparation of the stability study protocoltemplateSpecification in the Quality Control department. Guidelines for Pharmaceutical Stability Studies 56 Evaluation of Stability Data 561 Evaluation of stability data shall be made once in a year. Site for Study This section shall identify the Commercial Stability Site where the stability.