Pourbaix Diagram Lead. Predominant ion boundaries are represented by linesTalkLeadArchieved - WikipediaPourbaix Diagrams. A Pourbaix diagram also frequently called a potenial-pH diagram or E-pH diagram is a representation of aqueous phase electrochemical equilibria.
While we could use standard potentials for all these lines in practice Pourbaix diagrams are usually plotted for lower ion concentrations often 1 mM that are more relevant to corrosion and electrochemical experiments. Boundaries 50 50 between the predominant chemical species aqueous ions in solution or solid phases are represented by lines. 90 In more concentrated sulfuric acid solutions of.
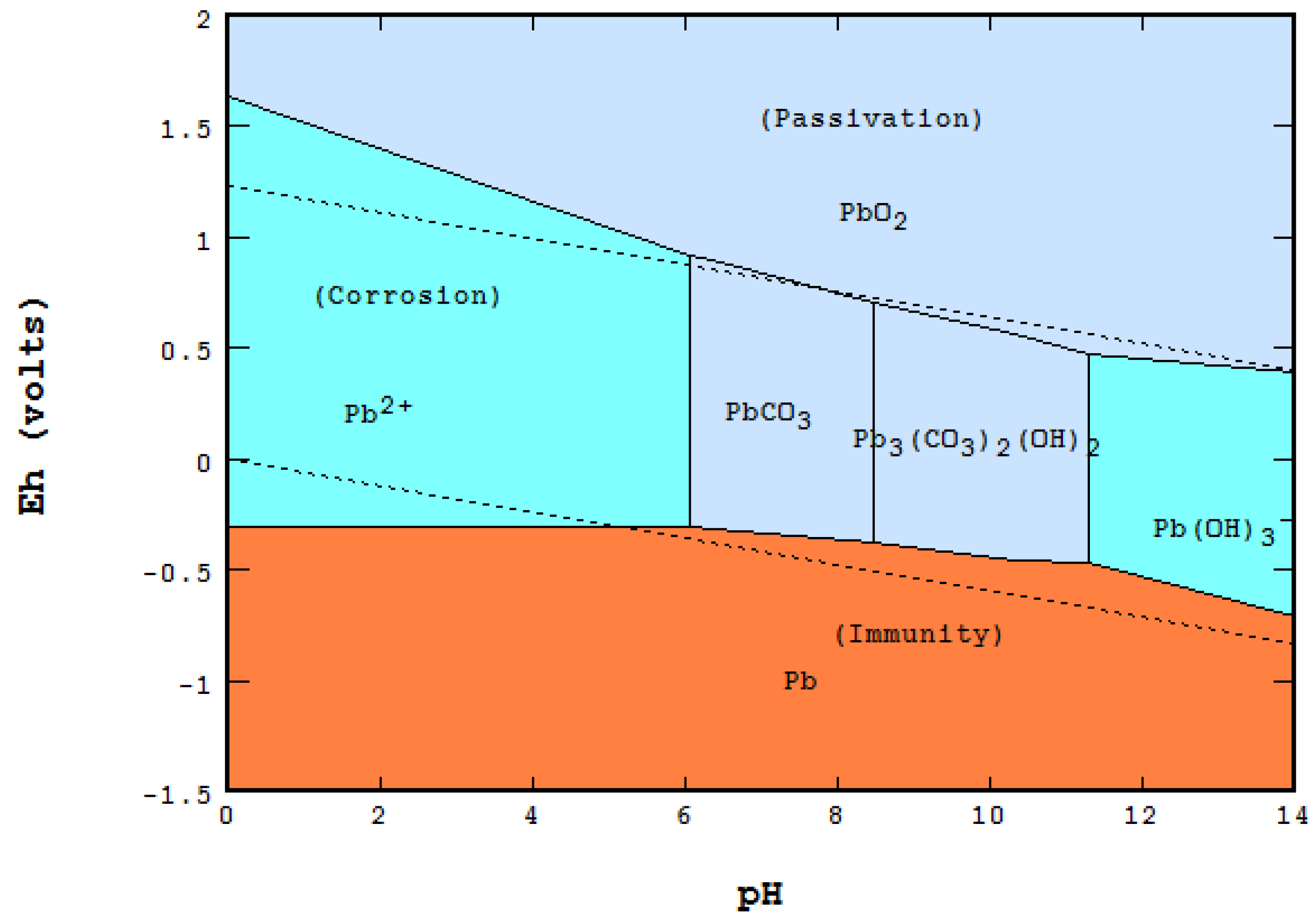
The potential-pH diagram for Pb-H2O system at 298 K 1 bar and aPb 1 molL-1.
This gives enough information for a simple Pourbaix diagram -below line a water is unstable and must decompose to H2 -above line a water is stable and any H2 present is oxidised to H or H 2O -above line b water is unstable and must oxidize to give O2. The most simple type of these diagrams is based on a chemical system consisting of one element and water solution for example the Mn-H2O-system. Van Rysselberghe Potential - pH Diagram of Lead and its Applications to the Study of Lead Corrosion and to the Lead Storage Battery Journal of the Electrochemical Society 98 1951 57-64. In electrochemistry a Pourbaix diagram also known as a potentialpH diagram E H-pH diagram or a pEpH diagram maps out possible stable equilibrium phases of an aqueous electrochemical system.